Koj puas tau tso lub raj mis dej hauv lub hnub kub li ob peb teev thiab hnov lub suab "ntxhi" me ntsis thaum koj qhib nws? Qhov no yog vim lub hauv paus ntsiab lus hu ua vapor pressure. Hauv kev siv tshuaj lom neeg, lub siab ua pa yog lub siab ua los ntawm cov phab ntsa ntawm lub thawv kaw thaum cov tshuaj lom neeg hauv nws evaporates (hloov mus ua roj). Txhawm rau nrhiav cov pa siab ntawm qhov ntsuas kub, siv Clausius-Clapeyron sib npaug: ln (P1/P2) = (ΔHchav/R) ((1/T2) - (1/T1)).
Kauj ruam
Txoj Kev 1 ntawm 3: Siv Clausius-Clapeyron Equation
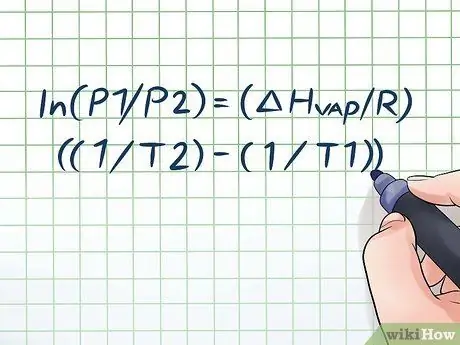
Kauj Ruam 1. Sau tus lej Clausius-Clapeyron
Cov mis siv los xam lub zog ua pa nrog kev hloov pauv huab cua lub sijhawm dhau los hu ua Clausius – Clapeyron equation (muaj npe tom qab kws kho mob lub cev Rudolf Clausius thiab Benoît Paul mile Clapeyron.) Cov lus nug ua pa nrov feem ntau pom hauv chav kawm physics thiab chemistry. Cov mis zoo li no: ln (P1/P2) = (ΔHchav/R) ((1/T2) - (1/T1)). Hauv cov mis no, qhov hloov pauv sawv cev:
-
Hchav:
Lub enthalpy ntawm vaporization ntawm cov kua. Qhov enthalpy no feem ntau tuaj yeem pom hauv lub rooj tom qab ntawm phau ntawv tshuaj lom neeg.
-
R:
Cov pa roj tiag tiag/thoob ntiaj teb, lossis 8.314 J/(K × Mol).
-
Q1:
Qhov ntsuas kub uas paub lub siab ua pa (lossis ntsuas kub thawj zaug).
-
T2:
Qhov kub uas lub siab ua pa tsis paub/xav kom pom (lossis qhov kub kawg).
-
P1 thiab P2:
Vapor siab ntawm qhov kub T1 thiab T2, feem.

Kauj Ruam 2. Nkag mus rau qhov hloov pauv uas koj paub
Clausius-Clapeyron equation zoo li nyuaj vim tias nws muaj ntau qhov sib txawv, tab sis nws tsis yog qhov nyuaj yog tias koj muaj cov ntaub ntawv raug. Feem ntau cov teeb meem huab cua yooj yim yuav teev ob qhov tseem ceeb ntawm qhov kub thiab ib qho txiaj ntsig ntawm qhov siab lossis ob qhov tseem ceeb ntawm qhov siab thiab ib tus nqi ntawm qhov kub - thaum koj xam qhov ntawd, daws qhov sib npaug no yooj yim heev.
- Piv txwv li, hais tias peb tau hais qhia tias peb muaj lub ntim ntim cov kua ntawm 295 K uas nws lub siab ua pa yog 1 cua (atm). Peb cov lus nug yog: Dab tsi yog qhov pa ntawm 393 K? Peb muaj ob qhov ntsuas kub thiab ib tus nqi siab, yog li peb tuaj yeem nrhiav lwm qhov txiaj ntsig siab siv Clausius-Clapeyron sib npaug. Los ntawm ntsaws rau hauv peb cov kev hloov pauv, peb tau txais ln (1/P2) = (ΔHchav/R) ((1/393) - (1/295)).
- Nco tseg tias, rau Clausius-Clapeyron equation, koj yuav tsum ib txwm siv tus ntsuas kub Kelvin. Koj tuaj yeem siv cov kev ntsuas siab ntev li ntev tau qhov txiaj ntsig rau P1 thiab P2 zoo ib yam.

Kauj Ruam 3. Nkag mus rau koj tus lej
Clausius-Clapeyron equation muaj ob qhov sib xws: R thiab H.chav. R ib txwm sib npaug 8.314 J/(K × Mol). Txawm li cas los xij, H.chav (enthalpy ntawm vaporization) nyob ntawm cov khoom uas nws ua pa siab koj tab tom nrhiav. Raws li tau hais los saud, feem ntau koj tuaj yeem pom qhov txiaj ntsig ntawm Hchav rau ntau yam tshuaj nyob tom qab ntawm phau ntawv tshuaj lom neeg lossis physics, lossis online (xws li, piv txwv li, ntawm no.)
-
Hauv peb qhov piv txwv, xav tias peb cov kua yog dej huv.
Yog tias peb saib hauv lub rooj qhov tseem ceeb ntawm Hchav, peb pom tias H.chav dej ntshiab yog li 40.65 KJ/mol. Txij li thaum peb tus nqi H yog hauv joules, thiab tsis yog kilojoules, peb tuaj yeem hloov nws mus 40,650 J/mol.
- Plugging hauv peb qhov ruaj khov, peb tau txais ln (1/P2) = (40,650/8, 314) ((1/393) - (1/295)).
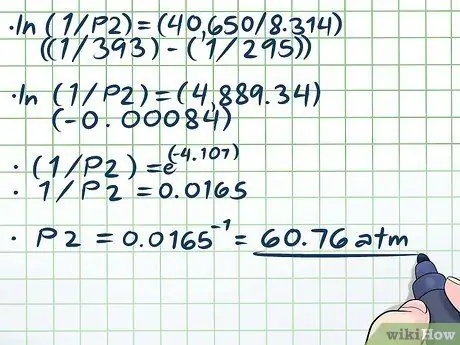
Kauj Ruam 4. Txhim kho qhov sib npaug
Thaum koj tau suav tag nrho cov kev hloov pauv hauv kab zauv tshwj tsis yog qhov koj tab tom nrhiav, npaj mus daws qhov sib npaug raws li cov cai ntawm algebra zoo tib yam.
-
Tsuas yog ib feem nyuaj ntawm kev daws peb cov kab zauv (ln (1/P2) = (40,650/8, 314) ((1/393) - (1/295))) yog daws qhov cav ntuj (ln). Txhawm rau tshem tawm lub cav ntuj, tsuas yog siv ob sab ntawm qhov kev ua zauv raws li nthuav tawm rau zauv zauv tas li e. Ua lwm yam lus, ln (x) = 2 → eln (x) = e2 → x = e2.
- Tam sim no, cia peb daws peb qhov sib npaug:
- ln (1/P2) = (40,650/8, 314) ((1/393) - (1/295))
- ln (1/P2) = (4889,34) (-0, 00084)
- (1/P2) = e(-4, 107)
- 1/P2 = 0.0165
-
P2 = 0.0165-1 = 60, 76 awm.
Qhov no ua rau kev nkag siab - hauv lub thawv kaw, nce qhov kub kom ze li 100 degrees (txog yuav luag 20 degrees siab dua qhov kub npau npau) yuav ua rau muaj pa ntau ntxiv, ua rau lub zog nrawm dua.
Txoj Kev 2 ntawm 3: Nrhiav Vapor Siab nrog Cov Tshuaj Tua Dej
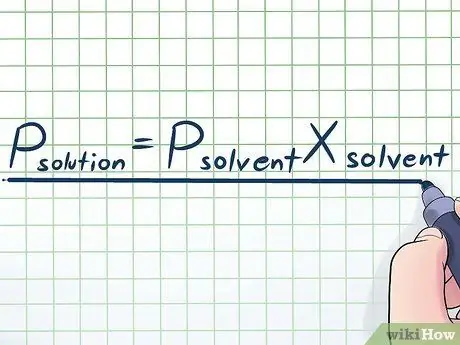
Kauj Ruam 1. Sau Raoult Txoj Cai
Hauv lub neej tiag, peb tsis tshua ua haujlwm nrog cov kua ntshiab - feem ntau, peb ua haujlwm nrog kua uas yog sib xyaw ntawm ntau yam tshuaj sib txawv. Qee qhov feem ntau siv sib xyaw ua ke yog ua los ntawm kev yaj me me ntawm qee yam tshuaj hu ua solute hauv ntau yam tshuaj hu ua kuab tshuaj los daws. Hauv cov xwm txheej no, nws yog qhov muaj txiaj ntsig kom paub qhov sib npaug hu ua Raoult's Law (muaj npe tom qab tus kws kho mob lub cev François-Marie Raoult), uas tau sau zoo li no: Pdaws= PebhnyavXhnyav. Hauv cov mis no, cov piv txwv sawv cev;
-
Pdaws:
Vapor siab ntawm tag nrho cov tshuaj (tag nrho cov ntsiab lus ua ke)
-
Phnyav:
Hnyav vapor siab
-
Xhnyav:
Mole feem ntawm cov kuab tshuaj
- Tsis txhob txhawj xeeb yog tias koj tsis paub cov ntsiab lus zoo li cov feem mole - peb yuav piav qhia lawv hauv ob peb kauj ruam tom ntej.

Kauj Ruam 2. Txiav txim siab qhov hnyav thiab daws hauv koj qhov kev daws teeb meem
Ua ntej koj tuaj yeem suav qhov siab ntawm cov kua sib xyaw, koj yuav tsum txheeb xyuas cov tshuaj koj tab tom siv. Raws li kev ceeb toom, kev daws teeb meem tau tsim thaum cov kuab tshuaj yaj hauv cov kuab tshuaj - cov tshuaj uas yaj tau ib txwm hu ua cov kuab tshuaj, thiab cov tshuaj uas ua rau nws yaj tas ib txwm hu ua cov kuab tshuaj.
- Cia peb ua haujlwm siv cov piv txwv yooj yim hauv ntu no los qhia txog cov ntsiab lus uas peb tham txog. Rau peb tus piv txwv, cia peb hais tias peb xav nrhiav lub zog ua pa ntawm qab zib qab zib. Kev lig kev cai, qab zib qab zib yog cov dej qab zib (1: 1 piv), yog li peb tuaj yeem hais tau li ntawd qab zib yog peb cov kuab tshuaj thiab dej yog peb cov kuab tshuaj.
- Nco ntsoov tias cov tshuaj lom rau sucrose (cov piam thaj hauv qab) yog C.12H22O11. Cov tshuaj formula no yuav yog ib qho tseem ceeb heev.
Kauj Ruam 3. Nrhiav qhov ntsuas kub ntawm cov tshuaj
Raws li peb pom hauv ntu Clausius Clapeyron saum toj no, qhov kub ntawm cov kua yuav cuam tshuam nws cov pa siab. Feem ntau, qhov kub siab dua, ntau dua qhov ua pa siab - raws li qhov kub nce, ntau cov kua yuav yaj thiab tsim ua pa, ua rau lub siab nyob hauv lub ntim.
Hauv peb qhov piv txwv, cia peb hais tias qhov ntsuas kub ntawm cov piam thaj hauv qab ntawm qhov no yog 298k ua (txog 25 C).
Kauj Ruam 4. Nrhiav cov pa ntawm qhov hnyav
Cov ntaub ntawv siv tshuaj lom neeg feem ntau muaj cov pa ntsuas qhov tseem ceeb rau ntau yam tshuaj siv thiab sib txuas, tab sis cov txiaj ntsig siab no feem ntau tsuas yog siv tau yog tias cov tshuaj muaj qhov kub txog 25 C/298 K lossis nws qhov kub npau npau. Yog tias koj qhov kev daws teeb meem muaj ib qho ntawm qhov ntsuas kub no, koj tuaj yeem siv tus nqi siv, tab sis yog tias tsis yog, koj yuav tsum nrhiav cov pa siab ntawm qhov kub ntawd.
- Clausius -Clapeyron tuaj yeem pab - siv qhov ntsuas qhov ntsuas pa thiab 298 K (25 C) rau P1 thiab T1 feem.
- Hauv peb qhov piv txwv, peb qhov sib tov muaj qhov kub txog 25 C, yog li peb tuaj yeem siv peb cov lus yooj yim siv. Peb paub tias ntawm 25 C, dej muaj lub zog ua pa ntawm 23.8 hli HG
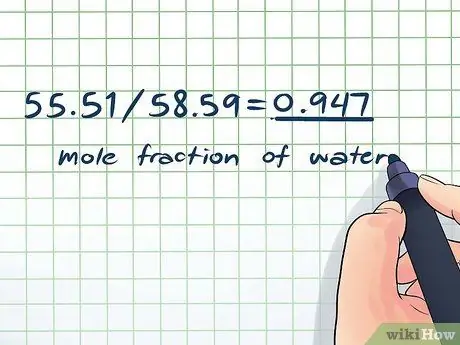
Kauj Ruam 5. Nrhiav cov feem me me ntawm koj cov kuab tshuaj
Qhov kawg uas peb yuav tsum tau ua ua ntej peb tuaj yeem daws qhov no yog txhawm rau nrhiav cov feem me me ntawm peb cov kuab tshuaj. Nrhiav cov mole feem yog ib qho yooj yim: tsuas yog hloov koj cov sib xyaw rau moles, tom qab ntawd pom qhov feem pua ntawm txhua qhov sib xyaw hauv tag nrho cov moles hauv cov khoom. Hauv lwm lo lus, cov feem me me ntawm txhua qhov sib xyaw yog sib npaug (moles ntawm compound)/(tag nrho tus naj npawb ntawm moles hauv cov khoom).
-
Xav tias peb daim ntawv qhia rau siv qab zib syrup 1 liter (L) dej thiab 1 liter sucrose (qab zib).
Hauv qhov no, peb yuav tsum pom tus naj npawb ntawm moles ntawm txhua qhov sib xyaw. Txhawm rau ua qhov no, peb yuav pom qhov hnyav ntawm txhua qhov sib xyaw, tom qab ntawd siv cov molar hnyav ntawm cov khoom kom hloov mus rau moles.
- Pawg (1 L dej): 1,000 grams (g)
- Pawg (1 L ntawm cov suab thaj nyoos): Kwv yees li 1,056, 8 g
- Moles (dej): 1,000 grams × 1 mol/18.015 g = 55.51 mol
- Moles (sucrose): 1,056, 7 grams × 1 mol/342.2965 g = 3.08 moles (nco ntsoov tias koj tuaj yeem pom cov molar loj ntawm sucrose los ntawm nws cov tshuaj lom neeg, C12H22O11.)
- Tag nrho cov moles: 55.51 + 3.08 = 58.59 mol
- Mole feem ntawm dej: 55, 51/58, 59 = 0, 947
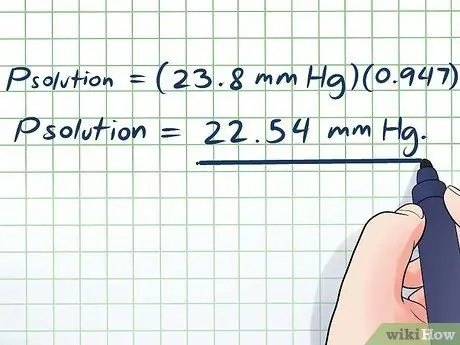
Kauj Ruam 6. Ua kom tiav
Thaum kawg, peb muaj txhua yam peb xav tau los daws peb li Raoult's Law equation. Qhov no yog qhov yooj yim heev: tsuas yog ntsaws rau hauv koj qhov txiaj ntsig rau qhov hloov pauv hauv qhov yooj yim Raoult Txoj Cai sib npaug ntawm qhov pib ntawm ntu no (Pdaws = PebhnyavXhnyav).
- Nkag mus rau peb qhov txiaj ntsig, peb tau txais:
- Pkev daws teeb meem = (23.8 hli Hg) (0, 947)
-
Pkev daws teeb meem = 22.54 hli Hg.
Qhov tshwm sim ua rau muaj kev nkag siab - hauv cov ntsiab lus mole, muaj piam thaj tsawg heev hauv cov dej ntau (txawm hais tias nyob hauv lub ntiaj teb tiag, ob qho khoom xyaw muaj tib lub ntim), yog li cov pa siab yuav tsuas qis me ntsis.
Txoj Kev 3 ntawm 3: Nrhiav Cov Vapor Siab hauv Cov Ntaub Ntawv Tshwj Xeeb

Kauj Ruam 1. Ceev faj nrog Tus Qauv Kub thiab Qhov Siab
Cov kws tshawb fawb feem ntau siv txheej txheej ntsuas kub thiab ntsuas qhov ntsuas raws li qhov yooj yim-rau-siv "tus qauv." Cov txiaj ntsig no hu ua Standard Kub thiab Siab (lossis STP). Cov teeb meem nqus pa feem ntau xa mus rau STP cov xwm txheej, yog li nws yog qhov tseem ceeb kom nco qab cov txiaj ntsig no. STP qhov tseem ceeb tau txhais raws li:
- Kub: 273, 15k ib / 0 C kev / 32f ua
- Siab: 760 hli Hg / 1 awm / 101, 325 kilopascals

Kauj Ruam 2. Rearrange Clausius-Clapeyron equation los nrhiav lwm yam kev hloov pauv
Hauv peb qhov piv txwv hauv Ntu 1, peb pom tias Clusius – Clapeyron equation muaj txiaj ntsig zoo rau kev nrhiav cov pa ua pa rau cov tshuaj ntshiab. Txawm li cas los xij, tsis yog txhua cov lus nug yuav nug koj kom nrhiav P1 lossis P2 - ntau tus yuav nug koj kom pom qhov ntsuas kub lossis qee zaum txawm tias tus nqi H.chav. Hmoov zoo, hauv cov xwm txheej no, tau txais cov lus teb yog yog ib qho teeb meem ntawm kev rov kho qhov sib npaug kom qhov sib txawv uas koj xav daws yog cais ntawm ib sab ntawm qhov sib npaug kos npe.
- Piv txwv li, hais tias peb muaj cov kua tsis paub nrog lub zog ua pa ntawm 25 torr ntawm 273 K thiab 150 torr ntawm 325 K, thiab peb xav nrhiav cov enthalpy ntawm kev ua pa ntawm cov kua no (ΔHchav). Peb tuaj yeem daws nws zoo li no:
- ln (P1/P2) = (ΔHchav/R) ((1/T2) - (1/T1))
- (ln (P1/P2))/((1/T2) - (1/T1)) = (ΔHchav/R)
- R × (ln (P1/P2))/((1/T2) - (1/T1)) = Hchav Tam sim no, peb nkag mus rau peb qhov tseem ceeb:
- 8, 314 J/(K × Mol) × (-1, 79)/(-0, 00059) = Hchav
- 8, 314 J/(K × Mol) × 3,033, 90 = Hchav = 25,223, 83 J/mol

Kauj Ruam 3. Laij cov pa ua pa ntawm cov kuab tshuaj thaum cov tshuaj tsim cov pa
Hauv peb qhov Raoult Law piv txwv saum toj no, peb cov kuab tshuaj, suab thaj, tsis siv lub zog ntawm nws tus kheej ntawm qhov kub ib txwm muaj (xav - thaum twg yog zaum kawg uas koj pom lub tais qab zib qhuav hauv koj lub txee sab saud?) Txawm li cas los xij, thaum koj qhov kev daws teeb meem tau ua evaporate, qhov no yuav cuam tshuam rau koj cov pa siab. Peb suav nrog qhov no los ntawm kev siv hloov kho dua tshiab ntawm Raoult Txoj Cai kev ua lej: Pkev daws teeb meem = (Pebsib xyawXsib xyaw) Lub cim sigma (Σ) txhais tau tias peb tsuas yog xav tau ntxiv txhua qhov kev sib zog ua pa ntawm cov sib txawv sib txawv kom tau txais peb cov lus teb.
- Piv txwv, hais tias peb muaj cov tshuaj ua los ntawm ob yam tshuaj: benzene thiab toluene. Tag nrho cov ntim ntawm cov tshuaj yog 12 milliliters (mL); 60 ml ntawm benzene thiab 60 mL toluene. Qhov ntsuas kub ntawm kev daws yog 25 ° C thiab cov pa ua pa ntawm txhua yam ntawm cov tshuaj no ntawm 25 ° C yog 95.1 mm Hg rau benzene thiab 28.4 mm Hg rau toluene. Nrog rau cov txiaj ntsig no, nrhiav cov pa siab ntawm qhov kev daws teeb meem. Peb tuaj yeem ua qhov no raws li hauv qab no, siv cov txheej txheem ntom ntom ntom ntom ntom ntom ntom ntom ntom ntom ntom, thiab cov pa ua kom muaj txiaj ntsig zoo rau peb ob yam tshuaj:
- Pawg (benzene): 60 mL = 0.060 L & lub sij hawm 876.50 kg/1,000 L = 0.053 kg = 53g ua
- Pawg (toluene): 0.060 L & zaus 866, 90 kg/1,000 L = 0.052 kg = 52g ua
- Mol (benzene): 53 g × 1 mol/78, 11 g = 0.679 mol
- Moles (toluene): 52 g × 1 mol/92, 14 g = 0.564 mol
- Tag nrho cov moles: 0.679 + 0.564 = 1.243
- Mole feem (benzene): 0.679/1, 243 = 0.546
- Mole feem (toluene): 0.564/1, 243 = 0.454
- Tshuaj: P.kev daws teeb meem = Pebcov benzeneXcov benzene + P.cov tolueneXcov toluene
- Pkev daws teeb meem = (95.1 mm Hg) (0, 546) + (28.4 mm Hg) (0, 454)
- Pkev daws teeb meem = 51.92 hli Hg + 12.89 hli Hg = 64, 81 hli Hg
Lub tswv yim
- Txhawm rau siv Clausius Clapeyron sib npaug saum toj no, qhov ntsuas kub yuav tsum ntsuas hauv Kelvin (sau ua K). Yog tias koj muaj qhov ntsuas kub hauv Celsius, tom qab ntawd koj yuav tsum hloov nws siv cov qauv hauv qab no: Tk 273 + TZSc
- Cov txheej txheem saum toj no tuaj yeem siv tau vim tias lub zog muaj qhov sib npaug raws li qhov siv cua sov. Qhov kub ntawm cov kua yog qhov ib puag ncig ib puag ncig uas cuam tshuam rau cov pa siab.







