Hauv cov tshuaj tiv thaiv tshuaj, teeb meem tsis tuaj yeem tsim lossis rhuav tshem, yog li cov khoom lag luam ntawm cov tshuaj tiv thaiv yuav tsum sib npaug ntawm cov tshuaj tiv thaiv kab mob hauv cov tshuaj tiv thaiv. Stoichiometry yog kawm txog kev sib raug zoo ntawm cov khoom hauv cov tshuaj tiv thaiv, uas suav nrog suav qhov hnyav ntawm cov tshuaj tiv thaiv thiab cov khoom hauv lawv. Stoichiometry yog kev sib xyaw ua lej thiab tshuaj lom neeg, thiab tau siv raws li ib txoj hauv kev yooj yim saum toj no, qhov teeb meem yeej tsis nce lossis txo qis hauv cov tshuaj tiv thaiv. Thawj kauj ruam los daws cov teeb meem chemistry yog ua kom sib npaug.
Kauj ruam
Ntu 1 ntawm 4: Ntsuas Kev Sib Txawv Tshuaj
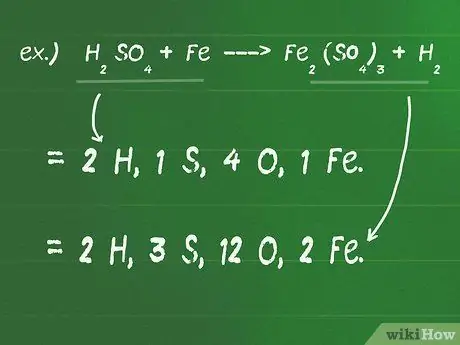
Kauj Ruam 1. Sau tus naj npawb ntawm cov atoms uas ua rau txhua qhov sib xyaw ntawm ob sab ntawm qhov sib npaug
Kev sib npaug tshuaj tuaj yeem pab koj txheeb xyuas cov atoms ntawm txhua lub hauv paus hauv cov tshuaj tiv thaiv. Hauv cov tshuaj tiv thaiv tshuaj, teeb meem tsis tuaj yeem tsim lossis rhuav tshem, yog li qhov sib npaug tau hais tias tsis sib xws yog tias tus lej (thiab hom) ntawm cov khoom sib cais ntawm ob sab ntawm qhov sib npaug tsis zoo ib yam.
- Tsis txhob hnov qab muab tus lej atoms los sib piv lossis tus lej hauv qab kab yog tias koj muaj.
- Piv txwv li, H.2YOG4 + Fe - Kev2(TSO4)3 + H.2
- Ntawm sab laug (reactants) sab ntawm qhov sib npaug muaj 2 H, 1 S, 4 O, thiab 1 Fe.
- Ntawm sab xis (khoom) sab ntawm qhov sib npaug muaj 2 H, 3 S, 12 O, thiab 2 Fe.
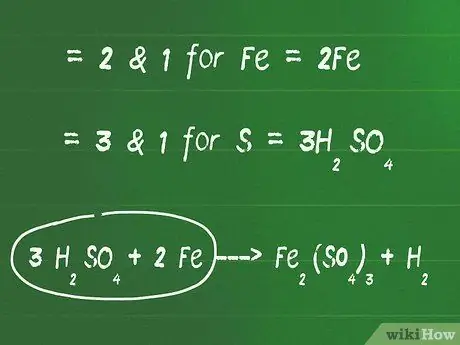
Kauj Ruam 2. Ntxiv coefficients nyob rau hauv pem hauv ntej ntawm cov ntsiab lus uas tsis yog oxygen thiab hydrogen kom sib npaug ntawm ob sab ntawm qhov sib npaug
Nrhiav qhov tsawg tshaj plaws ntawm cov ntsiab lus uas tsis yog oxygen thiab hydrogen kom sib npaug cov lej ntawm ob sab ntawm qhov sib npaug.
- Piv txwv li, qhov tsawg tshaj plaws sib txawv (LCM) ntawm 2 thiab 1 yog 2 rau Fe. Yog li, ntxiv tus lej 2 ua ntej ntawm Fe ntu ntawm sab laug kom sib npaug nws.
- LCM ntawm 3 thiab 1 yog 3 rau lub hauv paus S. Yog li, ntxiv tus lej 3 nyob rau hauv pem hauv ntej ntawm cov tshuaj H2YOG4 kom sib npaug sab xis thiab sab laug ntawm kab zauv.
- Nyob rau theem no, qhov sib npaug ntawm qhov piv txwv saum toj no yuav yog: 3 H.2YOG4 + 2 Fe - FeJ2(TSO4)3 + H.2
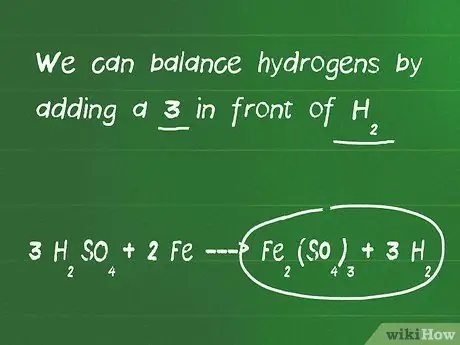
Kauj Ruam 3. Sib npaug hydrogen thiab oxygen atoms
Tus naj npawb ntawm hydrogen thiab oxygen atoms yog qhov sib npaug kawg vim tias lawv feem ntau muaj nyob hauv ob peb lub molecules ntawm ob sab ntawm qhov sib npaug. Hauv theem ntsuas ntawm qhov kev ua zauv no, tsis txhob hnov qab rov suav cov atoms tom qab koj tau ntxiv cov coefficients nyob rau hauv pem hauv ntej ntawm cov molecules.
- Hauv qhov piv txwv ntawm no, peb ntxiv tus lej 3 nyob rau hauv pem hauv ntej ntawm cov tshuaj H2YOG4, yog li tam sim no muaj 6 hydrogen atoms ntawm sab laug, tab sis tsuas yog 2 hydrogen atoms ntawm sab xis ntawm kab zauv. Tam sim no peb tseem muaj 12 cov pa oxygen nyob rau sab laug thiab 12 cov pa atoms ntawm sab xis, yog li cov pa oxygen sib npaug.
- Peb tuaj yeem sib npaug hydrogen atoms los ntawm kev ntxiv tus lej 3 nyob rau hauv pem hauv ntej ntawm H2.
- Qhov sib npaug kawg tom qab ntsuas yog 3 H.2YOG4 + 2 Fe - FeJ2(TSO4)3 + 3 H os2.
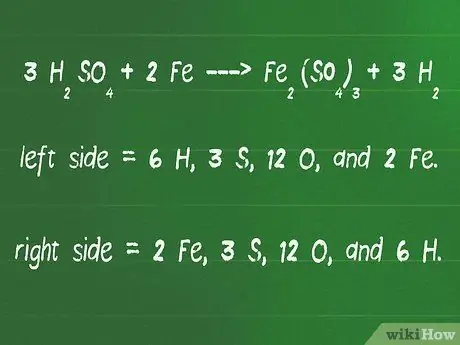
Kauj Ruam 4. Rov qab cov atoms ntawm ob sab ntawm qhov sib npaug kom paub tseeb tias lawv yog tus lej tib yam
Thaum ua tiav, rov suav dua thiab rov txheeb xyuas qhov sib npaug yog txoj cai. Koj tuaj yeem ua qhov no los ntawm kev ntxiv tag nrho cov atoms ntawm ob sab ntawm qhov sib npaug thiab ua kom ntseeg tau tias lawv zoo ib yam.
- Txheeb xyuas qhov sib npaug ntawm peb qhov sib npaug dua, 3 H.2YOG4 + 2 Fe - FeJ2(TSO4)3 + 3 H os2.
- Ntawm sab laug ntawm tus xub yog 6 H, 3 S, 12 O, thiab 2 Fe.
- Ntawm sab xis ntawm xub xub yog 2 Fe, 3 S, 12 O, thiab 6 H.
- Tus naj npawb ntawm atoms ntawm sab xis thiab sab laug yog zoo ib yam, yog li qhov sib npaug no twb sib npaug lawm.
Ntu 2 ntawm 4: Hloov Grams thiab Mol
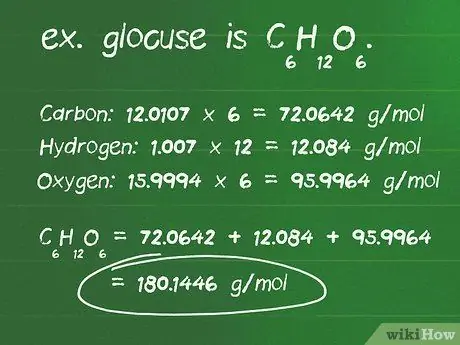
Kauj Ruam 1. Xam cov molar qhov hnyav ntawm qhov sib xyaw ua ke hauv grams
Molar loj yog tus naj npawb ntawm grams (g) hauv ib mole ntawm cov khoom sib xyaw. Chav tsev no tso cai rau koj hloov pauv grams thiab moles ntawm cov khoom sib xyaw. Txhawm rau suav qhov hnyav qhov hnyav, koj yuav tsum paub muaj pes tsawg tus qauv ntawm cov khoom nyob hauv qhov sib xyaw, nrog rau qhov hnyav atomic ntawm txhua lub hauv qhov sib xyaw.
- Nrhiav tus naj npawb ntawm atoms ntawm txhua lub ntsiab lus hauv cov khoom sib xyaw. Piv txwv, qabzib yog C6H12O6, thiab muaj li ntawm 6 carbon atoms, 12 hydrogen atoms, thiab 6 oxygen atoms.
- Tshawb nrhiav qhov hnyav atomic hauv grams ib mole (g/mol) ntawm txhua lub atom. Cov atomic pawg ntawm cov ntsiab lus uas ua rau cov piam thaj yog: carbon, 12.0107 g/mol; hydrogen, 1.007 g/mol; thiab oxygen, 15,9994 g/mol.
- Sib npaug txhua lub atom qhov hnyav los ntawm tus naj npawb ntawm atoms tam sim no hauv cov khoom sib xyaw. Cov pa roj carbon: 12.0107 x 6 = 72.0642 g/mol; hydrogen: 1.007 x 12 = 12,084 g/mol; oxygen: 15.9994 x 6 = 95.9964 g/mol.
- Qhov suav tag nrho cov khoom lag luam saum toj no yog qhov hnyav ntawm qhov sib xyaw. 72, 0642 + 12, 084 + 95, 9964 = 180, 1446 g/mol Los yog ua lwm yam lus, qhov hnyav ntawm ib qho piam thaj hauv cov piam thaj yog 180.14 grams.
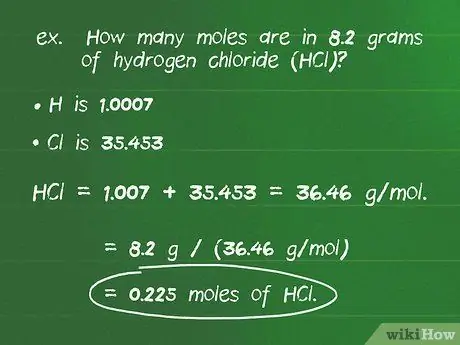
Kauj Ruam 2. Hloov qhov hnyav ntawm cov khoom sib xyaw mus rau moles siv cov pos hniav
Molar hnyav tuaj yeem siv los ua qhov hloov pauv, yog li koj tuaj yeem suav tus lej ntawm cov moles hauv ib tus lej ntawm cov qauv. Faib cov paub paub pawg (g) los ntawm cov molar pawg (g/mol). Ib txoj hauv kev yooj yim los tshuaj xyuas koj cov laij lej yog kom ntseeg tau tias cov koos pis tawj sib rho tawm thiab tawm tsuas yog cov moles.
- Piv txwv: muaj pes tsawg moles nyob hauv 8.2 grams hydrogen chloride (HCl)?
- Qhov atomic loj ntawm H yog 1.0007 thiab Cl yog 35.453 yog li qhov hnyav ntawm qhov sib xyaw saum toj no yog 1.007 + 35.453 = 36.46 g/mol.
- Sib faib cov lej ntawm cov sib xyaw los ntawm nws cov piam thaj muab: 8.2 g / (36.46 g / mol) = 0.225 mol HCl.

Kauj Ruam 3. Txiav txim siab qhov sib piv ntawm cov tshuaj tiv thaiv kab mob
Txhawm rau txiav txim siab tus nqi ntawm cov khoom lag luam tsim tawm hauv cov tshuaj tiv thaiv, koj yuav tsum txiav txim siab tus lej sib piv. Qhov sib piv ntawm cov pos hniav yog qhov sib piv ntawm cov sib txuas ua ke sib cuam tshuam, thiab tau qhia los ntawm cov coefficients ntawm cov sib txuas hauv cov tshuaj tiv thaiv uas tau sib npaug.
- Piv txwv li, qhov sib piv ntawm KClO yog dab tsi?3 nrog O2 hauv cov tshuaj tiv thaiv ntawm 2 KClO3 -2 KCl + 3 OO2.
- Ua ntej tshaj plaws, xyuas kom tseeb tias cov kab zauv saum toj no yog sib npaug. Tsis txhob hnov qab cov kauj ruam no lossis qhov sib piv ntawm cov hniav uas tau txais yuav tsis raug. Hauv qhov piv txwv no, tus nqi ntawm txhua ntu ntawm ob sab ntawm qhov sib npaug yog qhov sib npaug, yog li cov tshuaj tiv thaiv sib npaug.
- Qhov sib piv ntawm KClO3 nrog O2 yog 2/3. Koj tuaj yeem tso tus lej saum toj no thiab hauv qab, tsuav nws sawv cev rau qhov tsim nyog sib xyaw thoob plaws qhov teeb meem.

Kauj Ruam 4. Muab tus ntoo khaub lig los sib piv rau tus lej kom pom tus lej ntawm lwm cov tshuaj tiv thaiv
Txhawm rau xam tus naj npawb ntawm cov moles ntawm cov khoom tsim lossis xav tau hauv cov tshuaj tiv thaiv, koj tuaj yeem siv tus lej sib piv. Teeb meem tshuaj lom neeg feem ntau yuav nug koj kom txiav txim siab pes tsawg moles xav tau lossis tsim tawm hauv cov tshuaj tiv thaiv los ntawm qhov hnyav (grams) ntawm qee yam tshuaj tiv thaiv.
- Piv txwv li, hauv qhov sib npaug ntawm cov tshuaj tiv thaiv N2 + 3 H os2 - 2 NRH3 pes tsawg moles ntawm NH3 uas yuav tshwm sim los ntawm 3.00 grams N2 uas reacts nrog H.2 hauv qhov txaus?
- Hauv qhov piv txwv no, H.2 muaj nyob hauv qhov ntau txaus thiab koj tsis tas yuav suav lawv los daws qhov teeb meem.
- Ua ntej, hloov cov units ntawm grams N2 ua moles. Cov atomic loj ntawm nitrogen yog 14.0067 g/mol yog li cov molar hnyav yog N2 yog 28.0134 g/mol. Kev faib nruab nrab ntawm qhov hnyav thiab cov molar hnyav yuav muab 3.00 g/28.0134 g/mol = 0.107 mol.
- Xam tus piv hauv qhov teeb meem: NH3: Tsis2 = x/0,107 moles.
- Hla hla qhov sib piv no los ntawm qhov sib piv ntawm NH3 nrog N.2: 2: 1 x/0, 107 moles = 2/1 = (2 x 0, 107) = 1x = 0.214 moles

Kauj Ruam 5. Hloov tus naj npawb ntawm cov moles rov qab mus rau qhov hnyav uas siv cov molar loj ntawm qhov sib xyaw
Koj yuav siv cov piam thaj ntau dua, tab sis tam sim no xav tau qhov hnyav qhov hnyav los ua tus lej kom rov qab tus lej ntawm cov moles mus rau grams. Nco ntsoov siv qhov molar qhov hnyav ntawm qhov sib xyaw.
Molar pawg NH3 yog 17.028 g/mol Yog li 0.214 moles x (17,028 grams/mol) = 3.647 grams NH3.
Ntu 3 ntawm 4: Hloov Liters Cov Roj thiab Mol

Kauj Ruam 1. Tshawb xyuas seb qhov tshuaj tiv thaiv tau tshwm sim ntawm qhov ntsuas siab thiab ntsuas kub (STP)
STP yog txheej xwm txheej uas tso cai rau 1 mole ntawm cov pa zoo tshaj plaws kom ntim ntim ntawm 22.414 litres (l). Tus qauv ntsuas kub yog 273, 15 Kelvin (K) thiab tus qauv siab yog 1 cua (atm).
Feem ntau, hauv cov teeb meem nws yuav tau hais tias qhov kev tawm tsam tshwm sim ntawm 1 atm thiab 273 K, lossis hauv STP
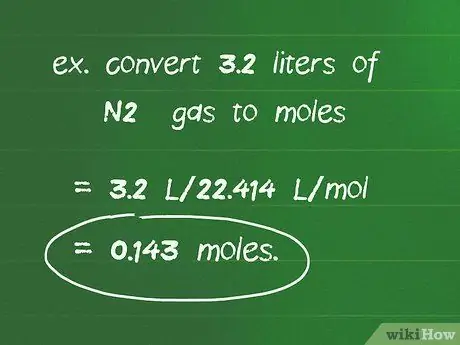
Kauj Ruam 2. Siv qhov hloov pauv ntawm 22,414 l/mol los hloov cov lej ntawm cov roj mus rau cov pa roj
Yog tias qhov tshuaj tiv thaiv tshwm sim nyob rau hauv STP cov xwm txheej, koj tuaj yeem siv 22.414 l/mol los xam tus naj npawb ntawm cov moles hauv cov ntim roj. Faib qhov ntim roj (l) los ntawm qhov kev hloov pauv no kom pom cov naj npawb ntawm cov moles.
Piv txwv li, kom hloov 3.2 litres ntawm N2 roj rau moles: 3.2 l/22, 414 l/mol = 0.143 mol.
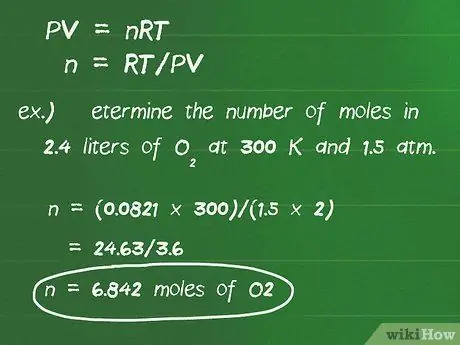
Kauj Ruam 3. Siv txoj cai lij choj siv roj zoo tshaj los hloov cov litres roj yog tias tsis nyob hauv STP cov xwm txheej
Yog tias qhov tshuaj tiv thaiv hauv qhov teeb meem tsis tshwm sim nyob rau hauv STP cov xwm txheej, koj yuav tsum siv txoj cai roj zoo PV = nRT los xam tus naj npawb ntawm cov moles hauv cov tshuaj tiv thaiv. P yog qhov siab hauv chav nyob hauv huab cua, V yog ntim hauv litres, n yog tus naj npawb ntawm moles, R yog txoj cai roj nkev tas li, 0.0821 l-atm/mol-degrees, thiab T yog qhov kub hauv degrees Kelvin.
- Qhov sib npaug no tuaj yeem rov kho dua los xam cov moles, los ua: n = RT/PV.
- Chav nyob ntawm cov pa tsis tu ncua tau tsim los tshem tawm txhua lwm yam kev hloov pauv hauv chav.
- Piv txwv, txiav txim siab tus naj npawb ntawm moles hauv 2.4 litres ntawm O2 ntawm 300 K thiab 1.5 atm. Plugging qhov hloov pauv mus rau qhov sib npaug, peb tau txais: n = (0.0821 x 300)/(1, 5 x 2) = 24, 63/3, 6 = 6, 842 moles O2.
Ntu 4 ntawm 4: Hloov Liters Liquids thiab Mol
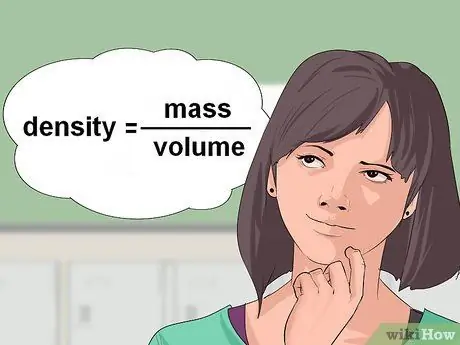
Kauj Ruam 1. Xam qhov ceev ntawm cov kua
Qee zaum, kev sib piv tshuaj muab qhov ntim ntawm cov tshuaj ua kua rau koj thiab thov kom koj suav tus lej lossis cov moles xav tau rau cov tshuaj tiv thaiv. Txhawm rau hloov qhov ntim ntawm cov kua rau grams, koj xav tau qhov ntom ntawm cov kua. Kev ntom tau qhia tawm hauv chav ntawm qhov hnyav/ntim.
Yog tias qhov ntom ntom tsis paub nyob hauv qhov teeb meem, tej zaum koj yuav tau saib nws hauv phau ntawv lossis hauv is taws nem

Kauj Ruam 2. Hloov lub ntim rau hauv milliliters (ml)
Txhawm rau hloov qhov ntim ntawm cov kua mus rau qhov hnyav (g), koj yuav tsum siv nws qhov ntom. Qhov ntom tau qhia hauv grams ib milliliter (g/ml), yog li qhov ntim ntawm cov kua yuav tsum tau qhia hauv milliliters los xam nws.
Nrhiav kom paub qhov ntim. Piv txwv li, cia peb hais hauv qhov teeb meem uas ntim ntawm H. yog paub2O yog 1 liter. Txhawm rau hloov nws mus rau ml, koj tsuas yog yuav tsum tau muab nws suav nrog 1000 vim tias muaj 1000 ml hauv 1 liter dej.

Kauj Ruam 3. Muab cov ntim ntim los ntawm qhov ceev
Thaum muab cov ntim (ml) los ntawm nws qhov ntom ntom (g/ml), cov units ml tau ploj thiab qhov tseem tshuav yog pes tsawg grams ntawm cov sib xyaw.
Piv txwv li, qhov ntom ntom H.2O yog 18.0134 g/ml. Yog tias qhov sib npaug tshuaj hais tias muaj 500 ml ntawm H2O, tus naj npawb ntawm cov gram hauv qhov sib xyaw yog 500 ml x 18.0134 g/ml lossis 9006, 7 g.
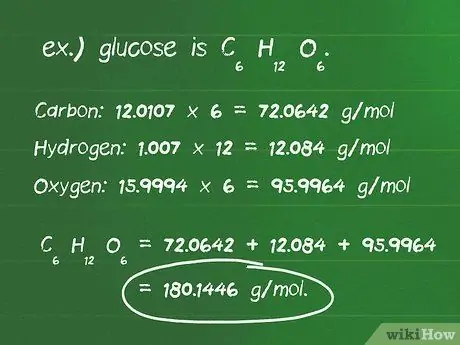
Kauj Ruam 4. Xam qhov hnyav ntawm cov tshuaj tiv thaiv kab mob
Molar loj yog tus naj npawb ntawm grams (g) hauv ib mole ntawm cov khoom sib xyaw. Chav tsev no tso cai rau koj hloov pauv cov units ntawm grams thiab moles hauv ib qho khoom sib xyaw. Txhawm rau suav qhov hnyav qhov hnyav, koj yuav tsum txiav txim siab pes tsawg lub molecules ntawm lub hauv paus nyob hauv ib qho chaw, nrog rau qhov hnyav atomic ntawm txhua lub hauv qhov sib xyaw.
- Txiav txim siab tus naj npawb ntawm atoms ntawm txhua lub ntsiab lus hauv cov khoom sib xyaw. Piv txwv, qabzib yog C6H12O6, thiab muaj li ntawm 6 carbon atoms, 12 hydrogen atoms, thiab 6 oxygen atoms.
- Tshawb nrhiav qhov hnyav atomic hauv grams ib mole (g/mol) ntawm txhua lub atom. Cov atomic pawg ntawm cov ntsiab lus hauv qabzib yog: carbon, 12.0107 g/mol; hydrogen, 1.007 g/mol; thiab oxygen, 15,9994 g/mol.
- Muab cov atomic loj ntawm txhua lub ntsiab los ntawm tus lej ntawm atoms tam sim no hauv cov khoom sib xyaw. Cov pa roj carbon: 12.0107 x 6 = 72.0642 g/mol; hydrogen: 1.007 x 12 = 12,084 g/mol; oxygen: 15.9994 x 6 = 95.9964 g/mol.
- Ntxiv cov txiaj ntsig sib npaug saum toj no kom tau cov suab thaj ntawm cov sib xyaw, uas yog 72, 0642 + 12, 084 + 95, 9964 = 180, 1446 g/mol. Yog li, qhov hnyav ntawm ib mole ntawm cov piam thaj yog 180.14 grams.

Kauj Ruam 5. Hloov cov naj npawb ntawm cov tshuaj sib xyaw ua cov moles uas siv cov pos hniav
Siv cov molar hnyav los ua qhov hloov pauv, koj tuaj yeem xam tus naj npawb ntawm cov moles tam sim no hauv tus lej ntawm cov piv txwv. Faib tus naj npawb ntawm cov gram (g) ntawm cov paub sib xyaw los ntawm cov molar loj (g/mol). Ib txoj hauv kev yooj yim los tshuaj xyuas koj cov laij lej yog kom ntseeg tau tias cov koos pis tawj sib rho tawm thiab tawm tsuas yog cov moles.
- Piv txwv li: muaj pes tsawg moles nyob hauv 8.2 grams hydrogen chloride (HCl)?
- Qhov atomic loj ntawm H yog 1.0007 thiab Cl yog 35.453 yog li qhov hnyav ntawm cov tshuaj sib xyaw yog 1.007 + 35.453 = 36.46 g/mol.
- Sib faib cov lej ntawm cov sib xyaw los ntawm cov molar muab: 8.2 g/(36.46 g/mol) = 0.225 mol HCl.







